Gaseous Laws and Ideal Gas Equation
Gaseous Laws and Ideal Gas Equation: Overview
This topic covers concepts, such as, Gas Laws, Boyle's Law, Pay-load Capacity of Hot Air Balloon & Vapour Density etc.
Important Questions on Gaseous Laws and Ideal Gas Equation
In a closed flask of 5 litres, 1.0 g of is heated from 300 K to 600 K. Which statement is not correct?
A bubble of air is underwater at temperature and the pressure 1.5 bar. If the bubble rises to the surface where the temperature is and the pressure is 1.0 bar, what will happen to the volume of the bubble?
Cyclopropane and oxygen at partial pressures torr and torr respectively are mixed in a gas cylinder. What is the ratio of the number of moles of cyclopropane to the number of moles of oxygen (Assume no reaction).
Under what conditions will a pure sample of an ideal gas not only exhibit a pressure of but also a concentration of ?
The correct value of the gas constant is close to:
If are pressure, volume, molar mass, temperature and gas constant respectively, then for an ideal gas, the density is given by
If are pressure, volume, molar mass, temperature and gas constant respectively, then for an ideal gas, the density is given by
Calculate the number of molecules in one litre of an ideal gas at temperature and pressure.
A vertical hollow cylinder of height is fitted with a weightless piston. The lower half is filled with an ideal gas and the upper half with mercury. The cylinder is now heated at , so that of the mercury comes out. Find the temperature at which it will happen assuming thermal expansion of to be negligible. (Give your answer up to two decimal)
Which one of these graphs for an ideal gas, the arrow indication is incorrectly marked?
A balloon contains of hydrogen. If the mass of the balloon without hydrogen is , its net lifting ability is (density of hydrogen , density of air =)
A mixture of and are trapped in a glass apparatus with a volume of . The pressure of total mixture was of Hg at . The sample was transferred to a bulb in contact with dry ice() so that are frozen out. When the sample returned to the normal value of temperature, the pressure was of Hg. The sample was then transferred to bulb in contact with liquid to freeze out . In the measured volume, the pressure was of Hg at original temperature. Mole of in mixture are .
So, the value of is......(as nearest integer)
The density of air at . Calculate the partial presser of gas assuming that air contains only gases.
The density of the gaseous mixture in a vessel at 2.1 atmosphere and 310 K is 1.35 g/litre. If a small pin-hole is made on the wall of the vessel, through which gases effuse, then which of the following is the correct composition of the gases He and effusing out initially?
A flask contains of oxygen at . (Assume gas is behaving ideally). The pressure inside the flask in bar is
(Given )
A bulb of constant Volume is attached to a very thin manometer tube as shown in the figure. Gas starts leaking through a small hole in the bulb causing change in pressure as :
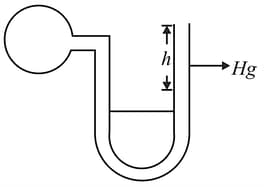
Where is constant and $P$ is pressure at any instant.
Initial height difference '' was and after was . Then the value of in
Select the incorrect statement(s):
Internal energy of mol of hydrogen of temperature is equal to the internal energy of mol of helium at temperature . The value of is
At STP, litre of a gas weighs . The vapour density of gas is -
Which of the following graphs correctly represent Charles' law?
